Innovate better and faster
The first 3-in-1 service integrating an efficient agile methodology, advanced statistical learning methods and support of experts.

What's easyQBD ?
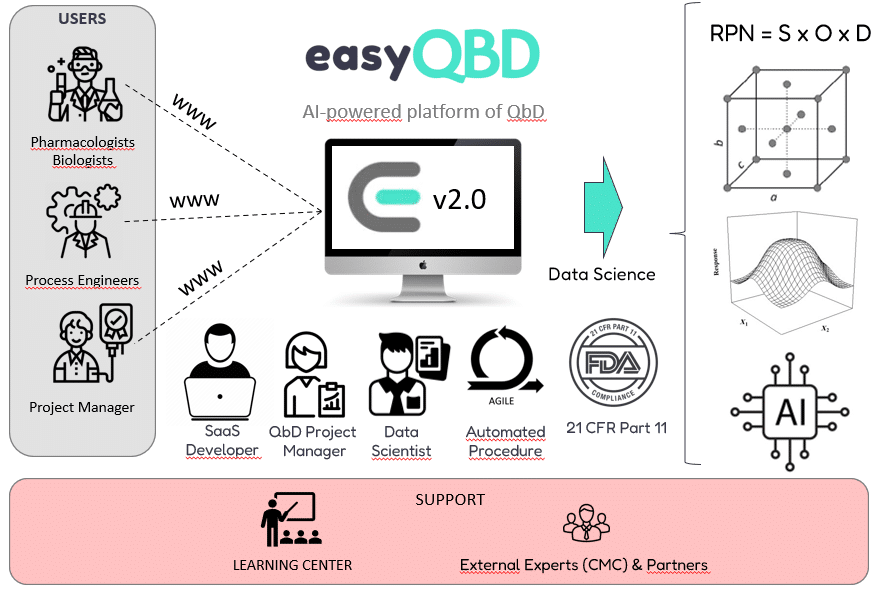
easyQBD is the first SaaS platform built on synergy between AI tools, agile methodology, and human expertise. It is designed to accelerate preclinical and clinical development phases by reducing their duration by an average of 30%.
easyQBD was initially developed for innovative startups, enabling them to utilize this development best practice affordably. At each stage, you are guided by an expert who helps you identify priority questions, fill in risk matrices, plan experiments, analyze data, and interpret results.
Today, easyQBD is utilized by various stakeholders to optimize their product composition, manufacturing process formulation, or analytical method performance.
Optimize the formulation of your product
By considering efficiency, safety, and quality requirements from the very beginning of the development process, this proactive approach maximizes the likelihood that the final product will best meet expected performance standards.
Derisk your drug development
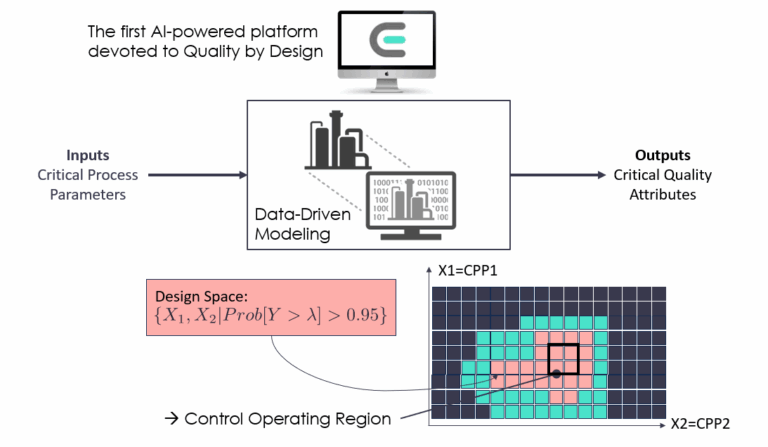
QbD emphasizes identifying and mitigating risks throughout the product's life cycle.
This is where experimental design tools, machine learning, and modeling come into play.
Data science is utilized to accomplish predictive and optimization tasks.
Speed up your market entry
QbD aligns with regulatory expectations for pharmaceutical development and manufacturing. By providing a systematic framework for documenting product and process understanding, QbD facilitates regulatory submissions and approvals. This can result in expedited review times and increased confidence from regulatory agencies, ultimately accelerating market entry and maximizing the commercial value of the drug project.
Benefits
Accelerate, de-risk, and enhance the value of your innovative product in full compliance with ICH guidelines Q8-Q14. ✅
Our clients love us
Thanks to depth attentiveness and comprehension of client’s needs, CYBERNANO is a reliable partner that delivers on time and complete outcomes. Highly competent in biological data modeling and analyses, the team shares their knowledge with pedagogy.
Céline BARRAUD
SANOFI PASTEUR
CYBERNANO has done a great job in assisting us to accelerate the optimization of our mRNA and lipid based nanoparticle platform. The CYBERNANO team combines excellent technical knowledge on data modelling with a customer oriented mindset and can do attitude. Highly recommended!
Stefaan De Koker
eTheRNA
Thanks to CYBERNANO expertise in bioproduction process optimisation Steminov has accelerated its drug candidate development. The QbD approach and platform of CYBERNANO is unique and a must have in any company working in bioproduction.
Julie Hutin
StemInov
CYBERNANO team has shown great professionalism, flexibility, and availability in the execution of their mission. CYBERNANO is attentive to the customer’s needs and has been able to suggest solutions compatible with industrial constraints through their meticulous data analysis
Joannah N’GOMPAZA-DIARRA
SEQENS
You want to save 20% of development time !?
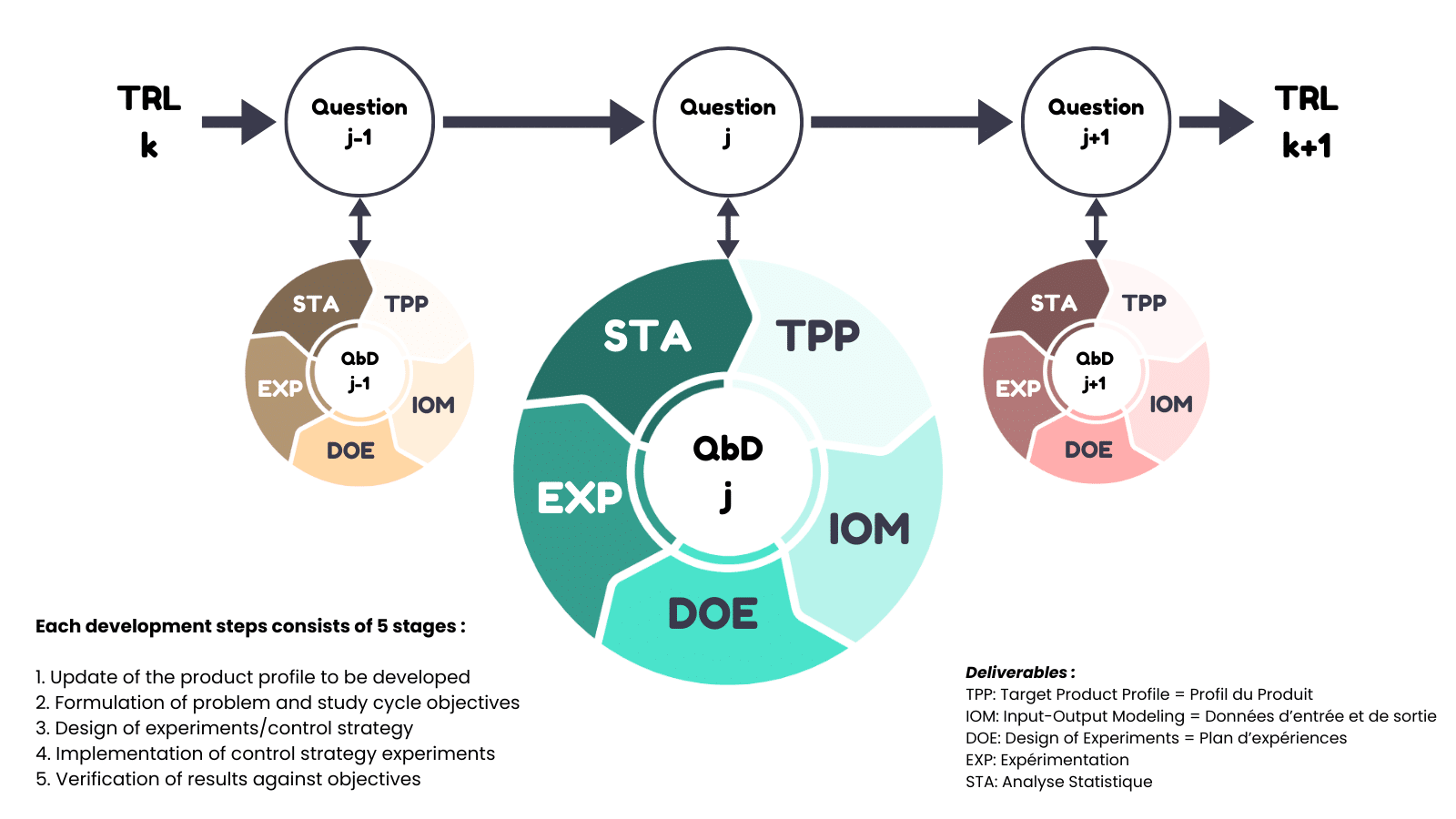
Quality by Design (QbD) enables the production of :
- key deliverables required by health authorities such as the (Q)TPP (Quality Target Product Profile),
- the list of critical input-output variables for the product and its manufacturing process,
- risk analyses,
- the design space,
- control strategy,
- Process Analytical Technology (PAT),
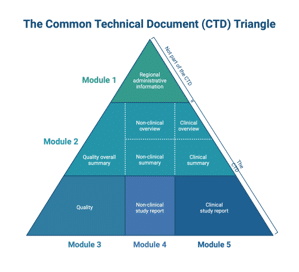
- process qualification, regulatory documentation (CTD modules 2 and 3),
- industrial transfer files.
This knowledge helps predict the optimal product formulation and the appropriate manufacturing process, as well as robust analytical procedures to accurately measure and monitor key process variables.